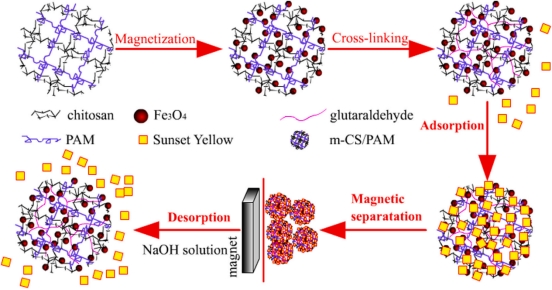
The water solubility in acid solution, relative low adsorption capacities and unsatisfactory separation performance limit application of traditional chitosan-based adsorbents in wastewater treatment. To break the limitation, a hydrophilic magnetic Fe3O4 embedded chitosan–crosslinked-polyacrylamide composites (abbreviated as m–CS–c–PAM) were prepared by a two–step method. The m–CS–c–PAM composites were systematically characterized using SEM, XRD, FTIR, VSM, TGA and BET. Sunset yellow (SY) was selected as model food dye to
investigate adsorption kinetics and thermodynamic parameters of food dye adsorption onto m–CS–c–PAM. Compared with magnetic Fe3O4/chitosan, m–CS–c–PAM can adapt to a wider range of pH (2–10) and resist the presence of inorganic salts. m–CS–c–PAM was proved to have high adsorption capacity (359.71 mg g− 1) for SY dye at 298 K, much higher than magnetic Fe3O4/chitosan and many reported adsorbents. Moreover, m–CS–c–PAM could be rapidly and efficiently separated from treated solution within 15 s by an external magnet and regenerated by NaOH solution. With its excellent adsorption capacity, pH–independent adsorption capability
for food dye, easy and convenient separation ability, satisfactory reusability, m–CS–c–PAM can be a promising material for food wastewater treatment.